Life History & Behaviour
Feeding
B. leachi is a filter feeder that removes a range of suspended material from the water column. Water enters the B. leachi via the buccal siphon, and flows through the pharynx and into the pharyngeal (branchial) basket (Pechenik, 2010). Lateral cilia on the gill silt are used to create water currents that move the suspended material (food) through the pharynx (Petersen, 2007, Ruppert et al., 2004). Food is then trapped on the mucous net that is secreted by the endostyle, and transported by the frontal cilia on the lining of the branchial basket until it reaches esophagus (Petersen, 2007, Ruppert et al., 2004). From here, food moves into the stomach where it undergoes extracellular digestion before entering the intestine. In the intestine, nutrition is absorbed and faeces is formed (Ruppert et al., 2004). Faeces is expelled as strings or pellets through the anus, into the atrium where it is pumped back out into the water column (Ruppert et al., 2004).
An experiment was conducted to investigate the feeding behaviour of B. leachi. According to Petersen, food particles are sieved by a set of tentacles, called the inhalent siphon aperture, as it enters the ascidian, preventing larger particles from passing through the pharynx (2007). The aim of the experiment was to find out the preferred algae size (1-2µm, 4-7µm or 7-20µm) of B. leachi. It was hypothesized that B. leachi should have a preference in algae size when it feeds. The preferred algae size of B. leachi was determined as the treatment that sees the biggest or fastest decrease in dry weight of algae. A total of 3 experiments were conducted.
Methods:
Colonies of B. leachi were collected and the number of functional zooids was counted. A functional zooid was defined as each buccal siphon with visible oral tentacles. Instant Algae®, a marine microalgae concentrate, of different sizes (1-2µm, 4-7µm and 7-20µm) was diluted to 0.03% dry weight and added to three beakers of seawater and mixed. 1ml water samples were taken and B. leachi was introduced. 1ml samples were then taken at regular time intervals for the entire duration of each experiment. At the end of experiment, B. leachi was removed and a final sample was taken.
Samples were then set to spin in a centrifuge machine at 3000g for 10 minutes. The supernatant was then replaced with 1ml of methanol before pellets were resuspended and storage in the fridge for 30 minutes. The resuspension was then set to spin at a top speed of 14800 rpm for 10 minutes before being transferred to a cuvette. An electro spectrometer was then used to measure the chlorophyll-a absorbance of each sample. The data collected was recorded. Table 2 describes how the three experiments were treated. Chlorophyll-a absorbance data will be extrapolated, converted to the dry weight of algae remaining in the beaker and plotted against time. Exponential trend lines were plotted on the final data to calculate the rate of change. By using the gradient of the trend lines, comparison will be made between the rates of change for each treatment.
Table 2: Description of each experiment
Results:
Figure 7: Percentage of dry weight of algae remaining in the beaker over time for experiment 1.
In experiment 1, the gradient of the trend line for the treatment with 1-2µm sized algae was 0.0183e-0.044x (r2 = 0.073) (Figure 7). The treatment with 4-7µm sized algae had a gradient of 0.0953e-0.019x (r = 0.27) while the treatment with 7-20µm sized algae had a gradient of 0.0449e-.0025x (r2 = 0.27) (Figure 7).
Figure 8: Percentage of dry weight of algae remaining in the beaker over time for experiment 2.
The gradient of the trend line for the treatment with 1-2µm sized algae was 0.0385e0.0058x (r2 = 0.011) for experiment 2 (Figure 8). For the treatment with 4-7µm sized algae, the gradient was 0.1624e-0.013x (r2 = 0.027) while the gradient was 0.0213e-0.037x (r2 = 0.62) for the treatment with 7-20µm sized algae (Figure 8).
Figure 9: Percentage of dry weight of algae remaining in the beaker over time for experiment 3.
In experiment 3, the gradient for the treatment with 1-2µm sized algae was 0.0247e0.0005x (r2 = 0.00012) (Figure 9). The gradient for the treatment with 4-7µm was 0.1066e-0.04x (r2 = 0.97) while the gradient for the treatment with 7-20µm sized algae was 0.0089e-0.004x (r2 = 0.035) (Figure 9).
Discussion:
This experiment aimed to investigate the preferred algae size of B. leachi and three experiments were conducted. Experiment 1 showed no difference in filter feeding rate across the three different algae sizes. In Experiment 2, the duration was shortened because in Experiment 1, the strongest trends were observed in the first 30 minutes. However, no differences were observed between the different algae sizes. In Experiment 3, the algae concentration was halved as failure to observe differences in Experiment 2 might be due to over saturation. Experiment 3 revealed that the treatment with 4-7µm sized algae had a higher and more consistent filter-feeding rate as compared to the two other treatments with 1-2µm and 7-20µm sized algae.
The results from Experiment 3 seem to show that 4-7µm sized algae were preferred by B. leachi. However, one should be hesitate to draw definitive conclusions from these results.
First, the differences in filter feeding rate cannot be statistically compared against each other because there was only one replicate. As such, these differences might be due to chance. Therefore, more replicates will be needed to substantiate these findings.
Second, the algae used might not have been what B. leachi used to. Therefore, B. leachi might not have been feeding at all during the experiments. In addition, only three size ranges of algae were used. A limitation of this is that the actual preferred size of algae of B. leachi might not have been represented. Hence, the lack of feeding across all the experiments might be due to the algae type used.
Third, across all three experiments, there were fluctuations in all treatment samples. A possible reason for these observed fluctuations might be technical variation such as measurement errors. One example would be the sharing and reusing of disposable cuvettes. This can lead to contamination of the samples and inaccurate electro spectrometer readings.
Fourth, the colonies of B. leachi might have been stressed from being out of the water during transfer from storage tanks to treatment beakers. In addition, the colonies of B. leachi were not given time to acclimatize before experiment was started. As a result, feeding might have been temporarily stopped. This can be overcome by allowing time for acclimatization.
In conclusion, an indication of a preference was found in the 4-7µm sized algae treatment of Experiment 3. However, this should be taken with a pinch of salt because of the lack of replicates, type of algae used, technical variation and animal stress. Future studies build on these weaknesses and can explore other experiments and ways of investigating the feeding preferences of B. leachi.
Reproduction
B. leachi can reproduce both sexually and asexually simultaneously (Ruppert et al., 2004, Satoh, 1994). Scientists have found that colonial ascidians like B. leachi, have to have a asexual phase in their life cycle in order for them to have sexual reproduction (Satoh, 1994). Zooids developed from an egg (oozooid) is unable to reach sexual maturity and only propagates asexually, while zooids developed from a bud (blastozooid) can reproduce both sexually and asexually (Ruppert et al., 2004, Satoh, 1994). When B. leachi undergoes winter quiescence, reproduction rate is reduced (Burighel et al., 1976).
The mode of sexual reproduction in B. leachi is ovoviviparous (Satoh, 1994) and self fertilization is common (Ruppert et al., 2004). B. leachi is a hermaphrodite with paired gonads on each side of the body wall. B. leachi produces yolky eggs that when fertilized, develop in the brood pouch (Ruppert et al., 2004, Satoh, 1994). The embryos undergo lecithotrophic development into a tadpole like larvae that swims out into the water column from the atrial siphon (Ruppert et al., 2004, Satoh, 1994). All the key chordate features are expressed in the tadpole stage (Ruppert et al., 2004).
When a larva settles, forms a single zooid that undergoes asexual reproduction and clones a colony (Manni et al., 2007). The blastozooids produced from asexual reproduction are genetically identical to its parent (Tiozzo et al., 2008). B. leachi reproduces asexually by going through budding and strobilation (Satoh, 1994). There are 2 types of budding: propagative and survival (Satoh, 1994). Propagative budding is the basis of colony growth while survival budding is passive and ensures the survival of the colony in adverse environmental conditions (Satoh, 1994).
Metamorphosis
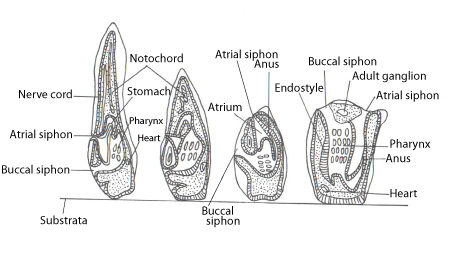
Figure 10: (from left to right) illustration of a tadpole like larva of B. leachi undergoing metamorphosis to a juvenile oozooid. Adapted from Ruppert et al. 2004. Invertebrate zoology: a functional evolutionary approach.
The larva of B. leachi does not feed as its gut and associated structures are nonfunctional (Ruppert et al., 2004). When the negatively phototrophic larva (Kott, 2005) is ready to settle, it attaches to the substratum with 3 anterior adhesive papillae (Manni et al., 2007, Ruppert et al., 2004). Metamorphosis then starts with the notochord becoming flaccid and the tail retracting (Figure10) (Ruppert et al., 2004). As the tail retracts, the notochord, dorsal hollow nerve cord, swimming muscles and endodermal rudiments are lost (Ruppert et al., 2004, Tiozzo et al., 2008). The region between the adhesive papillae and the buccal siphon undergoes rapid growth and causes the buccal and atrial siphons to face upwards (Ruppert et al., 2004). The anus and the pharynx become enclosed by the atrium that expands while the number of gill silts increase (Ruppert et al., 2004). Finally, metamorphosis is completed when the larval cuticle is shed and the siphons open to the exterior environment so that it can start feeding (Ruppert et al., 2004, Tiozzo et al., 2008).
Movement
The colonial form of B. leachi is a sessile organism that stays attached to its substratum (NIMPIS, 2012b). The free-living larvae produced from sexual reproduction uses a propulsive tail to manoeuvre in the water column until it is ready to settle and attach to a substratum (Ruppert et al., 2004).
Glossary:
Chlorophyll-a: a type green pigment used in plants for photosynthesis.
Electro spectrometer: a machine that measures the amount and length of wavelengths that can pass through a liquid.
Lecithotrophic: lives of the yolk (nutrients) from the egg.
Metamorphosis: when an organism undergoes rapid and conspicuous change; generally between larva and adult form.
Negatively phototrophic: sensitivity to light that causes it to move away from the light; avoid the light.
Notochord: flexible rod like structure of mesodermal cells found in embryos of all chordates.
Ovoviviparous: eggs develop (and hatch) within the adult's body, giving birth to live young.
Papillae: a nipple like (shaped) structure
|